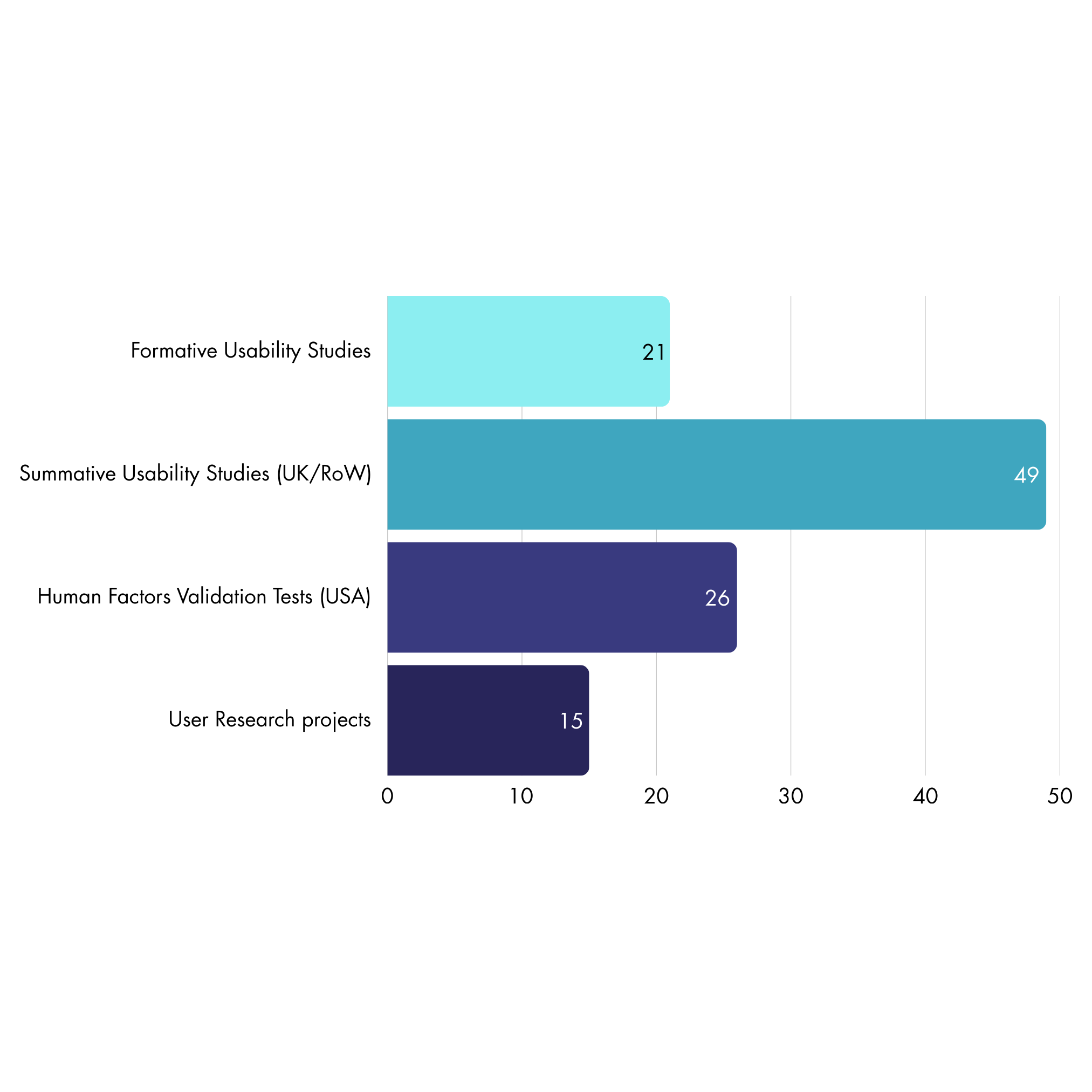Pharmaceutical Human Factors Engineering
PROJECT HISTORY
✓ Completion of over 160 projects.
✓ Projects performed in over 10 countries.
✓ Customers range from world's largest MedTech organisations to start-ups.
✓ Every project successful!
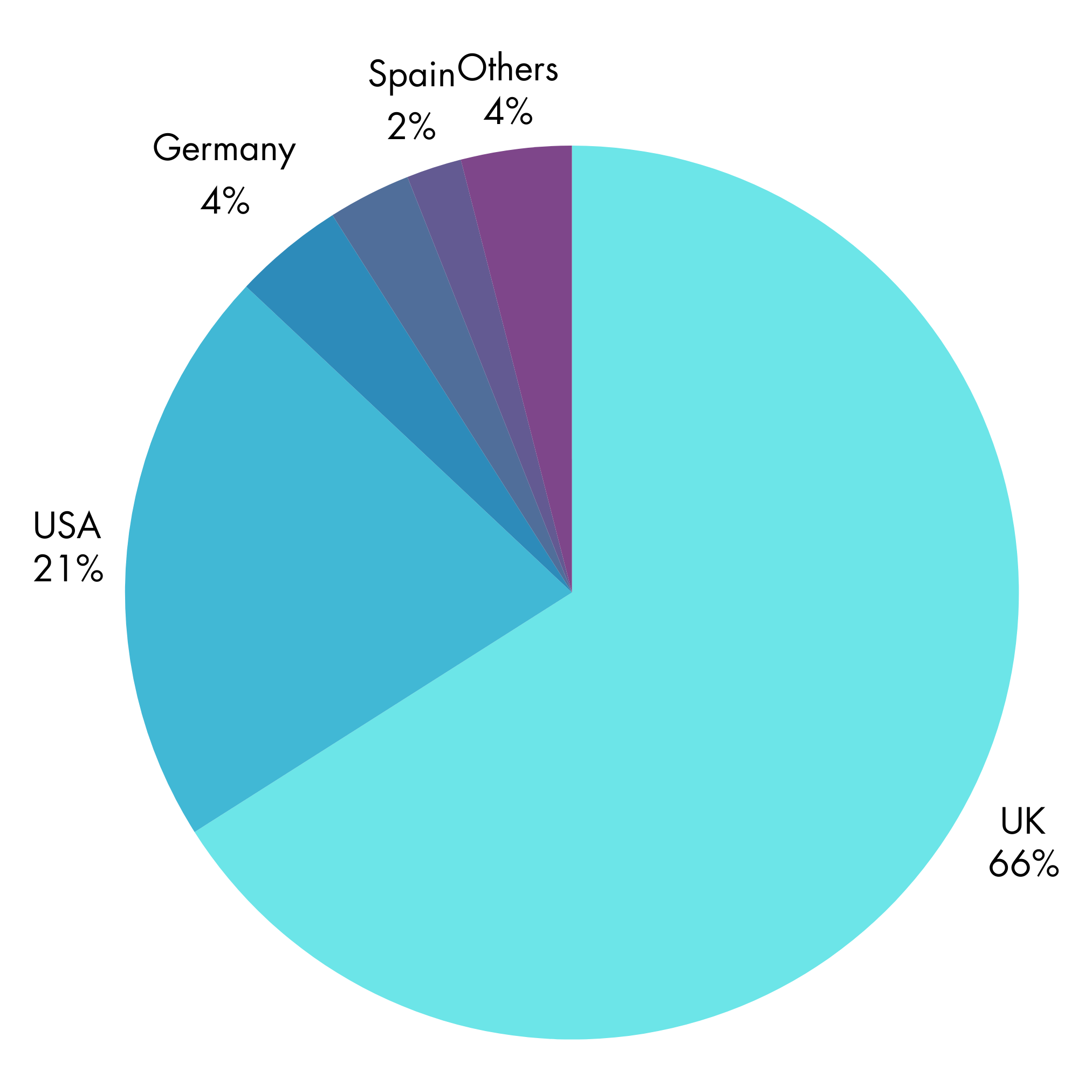
OUR CUSTOMERS
75+ global clients
55% of clients return to use THAY PHARMA.
100% are in healthcare.
We have conducted:
21 Formative Usability Studies
49 Summative Usability Studies (UK/RoW)
26 Human Factors Validation Tests (USA)
12 MDR / Usability Assessments (EU)
15 User Research projects
COMPLIANCES PERFORMED TO:
-
Before developing a solution, it is often prudent to verify the design inputs, to ensure what Marketing and Sales teams state is what is needed, required or desired by users. The following are some services we offer that can help you to confirm the design and development inputs to ensure the correct outcomes at the end of the process.
Ethnography through ethnographic research studies.
Contextual Enquiry (interviews) with users and interactors.
Comparative Human Factors Studies where many “off-the-shelf” solutions are available.
Online research to formulate ‘Use Specification’ documents and initial usability requirements.
-
Predicting the future use of your pharmaceutical product is easy when you can generate data to statistically support theory. The habits and use processes users frequently use are well known, and often predictable.
When you have prediction data, you can ensure Use-related Risk Analyses are accurate, design solutions are efficient and effective and instructional or training materials work well based upon real world data. All these elements create the user experience which can be the difference between a successful pharmaceutical product or one that just meets the clinical need.
We offer the following services to enable prediction to become a reality:
→ Use-focused Surveys
→ Use-focused Formative Usability Studies
→ Use-focused Existing & New Data Analysis
→ Use-focused Remediation Usability Testing
→ AI Data Extrapolation & Trending
-
It is regulated that every medical device, system or software have user documents that are supplied that explain the intended use, the clinical application and the lifecycle all the way through to disposal or recycling. These can be in the form of an Instructions for Use, a User Manual, a Quick Guide, an e-IFU, a website, an App or simply packaging and labelling. These are important to ensure safe and effective use and as such should be tailored to the intended users to make our lives as easy as possible.
THAY Medical design and develop user documents to focus on the intended users and then verify and validate them with actual users in studies and tests to ensure they are safe and effective and usable. We perform:
→ The design and development of User Documents for all medical and pharmaceutical products
→ Readability Studies to the EMA (European Medicines Agency) regulations for pharmaceutical products
→ Formative-level Knowledge Task Assessment (verification) Comprehension Studies
→ Summative-level Knowledge Task Assessment (validation) Comprehension Tests
-
With all pharmaceutival products, the proof they are safe and effective lies wthin generating realistic evidence - high quality evidence. This is done by performing user testing. It is also called ‘Human Factors Testing’ or ‘Usability Testing’ and is regulated by process in many parts of the world. For pharmaceutical products, the main regulations and standards are focused on the US, European, UK and Chinese territories to enable market authorisations in these countries.
THAY Medical work to all regulations and standards and these form a significant part of our ISO13485 certified Quality Management System (which is certified for ‘Human Factors Engineering’ only). We only perform our services compliantly and often exceed the requirements stated in these regulations and standards.
We offer the following Human Factors Tests and Usability Studies:
→ Pharmaceutical product user research to determine all specifications, requirements and user needs
→ Pharmaceutical product Comparative Human Factors Studies (to meet US FDA Guidance)
→ Pharmaceutical product Comparative Usability Studies (to meet EU MDR & EMA Regulations)
→ Pharmaceutical product Formative Usability Studies (to meet IEC/ISO 62366-1 standards)
→ Pharmaceutical product Human Factors Validation Tests (to meet US FDA Guidance)
→ Pharmaceutical product Summative Usability Studies (to meet IEC/ISO 62366-1 standards)
Our Pharmaceutical Customers